How does Deviation Management Differ from Risk Management?

Risk management and deviation management are important quality management processes, each addressing different and unique quality and compliance aspects. Risk Management proactively identifies, assesses, and mitigates potential uncertainties that could adversely affect an organization's quality objectives. The process aims to anticipate risks and implement strategies to minimize their impact.
Deviation Management is a reactive approach that identifies, documents and corrects unexpected events or departures from established procedures and standards during operations. It focuses on addressing issues post-occurrence to prevent recurrence and ensure compliance.
Neglecting these critical processes can lead to substantial financial and reputational damages. For example, the average cost of a manufacturing deviation is estimated at $100,000 per incident, with some lost batches costing companies between $250,000 and $500,000 each. Moreover, poor data quality, often stemming from inadequate deviation management, can result in 20% of revenue losses, equating to approximately $3.1 trillion in the U.S. alone.
Qualityze Risk Management Software and CAPA Management Software enables organizations to manage risks and deviations effectively. These tools facilitate compliance with regulatory standards, enhance operational efficiency, and support achieving organizational goals by providing structured frameworks for identifying, assessing, and mitigating risks and deviations.
Understanding Risk Management
Risk Management is a systematic process that identifies, evaluates, and addresses potential challenges that can impact an organization. Key components include:
-
Risk Identification: Recognizing potential threats that could hinder objectives.
-
Risk Assessment: Analyzing the likelihood and impact of identified risks.
-
Risk Mitigation: Developing strategies to reduce or eliminate the impact of risks.
-
Risk Monitoring: Continuously overseeing risk factors and the effectiveness of mitigation strategies.
Effective risk management is crucial for organizational resilience. Studies indicate that only 33% of risk leaders plan to increase spending on risk management, highlighting a potential gap in proactive risk mitigation efforts.
Understanding Deviation Management
Deviation Management focuses on handling deviation from established standards or procedures. It includes:
-
Deviation Identification: Detecting anomalies or non-conformities in processes.
-
Deviation Documentation: Recording details of the deviation for analysis.
-
Investigation and Root Cause Analysis: Determining the underlying causes of the deviation.
-
Corrective and Preventive Actions (CAPA): Implementing measures to correct the deviation and prevent future occurrences.
Effective deviation management is essential in FDA-regulated industries to maintain compliance and product quality.
|
Aspect |
Risk Management |
Deviation Management |
|
Purpose |
Prevents potential risks. |
Corrects existing deviations. |
|
Timing |
Proactive approach. |
Reactive approach. |
|
Focus |
Future uncertainties. |
Past or current failures. |
|
Outcome |
Reduces likelihood of issues. |
Prevents recurrence of issues. |
The Cost of Neglecting Risk and Deviation Management
Failing to implement risk and deviation management processes leads to significant consequences:
-
Financial Losses: Deviations can increase project costs, especially requiring rework or additional testing.
-
Reputational Damage: Unmanaged risks and deviations can lead to product recalls or compliance issues, tarnishing an organization's reputation.
-
Operational Inefficiencies: Unaddressed deviations can cause process inefficiencies, leading to delays and increased operational costs.
Field Safety & Recall Management Software: Bridging the Gap Between Risk and Deviation Management
Product quality and patient safety are top priorities in regulated industries like pharmaceutical and medical devices. To maintain compliance and trust, organizations must manage potential threats and actual failures equally.
While risk and deviation management operate at different stages of the quality lifecycle, they often intersect—especially when a product reaches the field. This is where Field Safety and recall Management Software becomes essential.
The software acts as a centralized system to track, manage, and resolve field-related product issues, whether from predicted risks or unplanned deviations. It connects the dots between quality events, customer complaints, adverse event reports, and regulatory requirements. If a known risk becomes a field reality, the system can trigger automated actions, such as issuing safety notices or initiating a product recall. Likewise, the software ensures a structured response with traceable documentation and communication workflows if a deviation escalates to a field incident.
By bridging proactive and reactive processes, the software supports full lifecycle management of product quality and safety. It helps organizations:
-
Monitor and manage product performance in the field
-
Detect trends and recurring issues early
-
Link field events to root causes in manufacturing or supply chain
-
Automate recall and correction procedures
-
Stay compliant with global regulatory bodies (FDA, EMA, MHRA, etc.)
The key difference between risk management and deviation management lies in timing and intent—one seeks to prevent issues while the other responds. However, both play crucial roles in protecting end users and preserving brand reputation.
Qualityze Risk and CAPA Management Software
Qualityze EQMS Software offers comprehensive solutions for managing risks and deviations, ensuring organizations meet compliance requirements and achieve operational excellence.

Quality Risk Management software anticipates and pre-empts challenges from escalating into risks. It is built on Salesforce, one of the safest platforms, and is flexible, scalable, intuitive, and easy-to-use.
Field Safety and recall Management Software doesn’t just handle recalls—it unifies risk and deviation data to create a smarter, faster, and more compliant response system. This transforms field safety from a reactive necessity into a proactive strategic advantage.
Features of Qualityze Risk Management Software
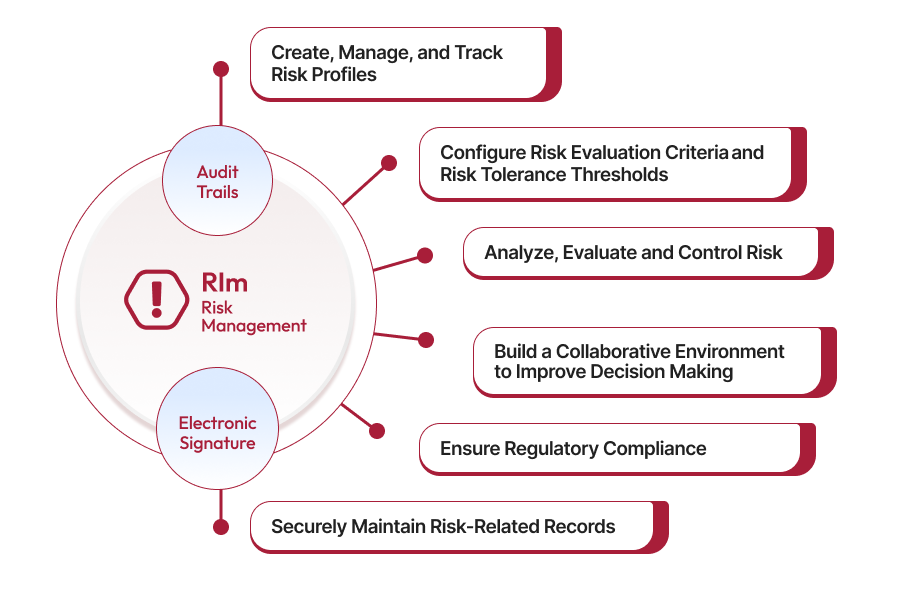
Risk Profile Management enables organizations to create detailed risk profiles, including cause descriptions and risk sources, facilitating thorough risk assessments.
-
AI-Powered Risk Workflow Automation: Utilizes artificial intelligence to automate risk detection and resolution processes, enhancing efficiency and accuracy.
-
Real-Time Collaboration: Facilitates seamless collaboration among teams, regardless of location, through cloud-based infrastructure.
-
360-Degree Visibility: Provides comprehensive dashboards and reports, offering a holistic view of quality metrics and compliance status.
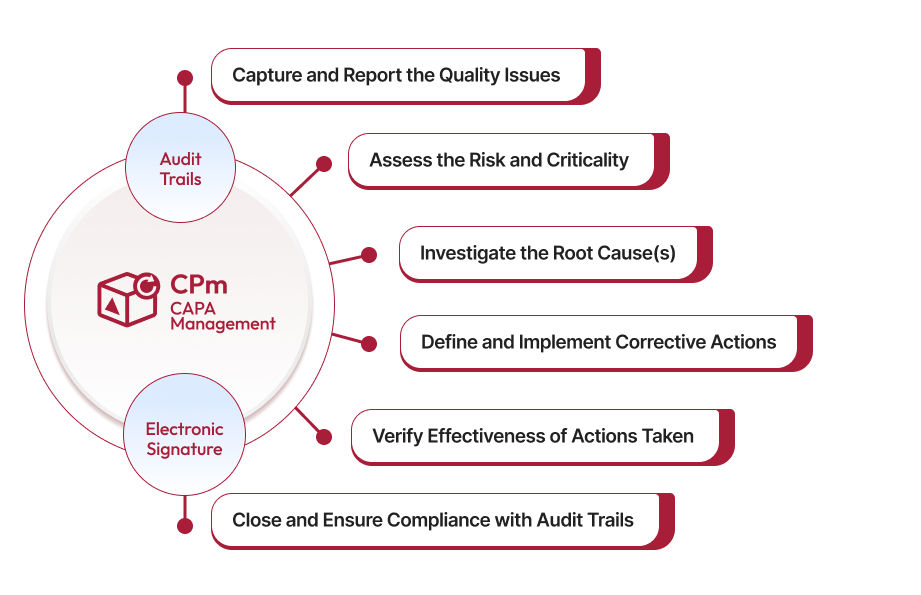
Features of Qualityze CAPA Management Software
-
Personalized CAPA Dashboards: These offer real-time insights into CAPA processes, enabling the tracking of key metrics and identification of bottlenecks.
-
Root Cause Analysis and Risk Assessment: Facilitates thorough investigations into non-conformities, ensuring effective corrective and preventive actions.
-
Integration with QMS: Seamlessly integrates with existing Quality Management Systems, streamlining processes and ensuring compliance.
Conclusion
Distinguishing between Risk Management and Deviation Management is vital for organizations aiming to maintain quality and compliance. While risk management proactively addresses potential threats, deviation management focuses on rectifying and preventing the recurrence of process anomalies. Neglecting these areas can lead to significant financial losses and reputational harm. Implementing robust solutions like Qualityze Risk and CAPA Management Software empowers organizations to manage risks and deviations effectively, ensuring compliance and operational excellence.
Wacth this video to get additional information -
- Difference_Between_Risk_Management_and_Deviation_Management
- Risk_Management_vs._Deviation_Management
- Risk_and_deviation_management
- Risk_Management_&_Deviation_Management_Software
- Field_Safety_&_Recall_Management_Software
- risk_management_software
- capa_management_software
- capa_software
- enterprise_risk_management_software
We are excited to announce the **launch of the Sharkbow Marketplace!** 🎉 Now you can:
- 🛍️ List and sell your products – Open your own store easily.
- 📦 Manage orders effortlessly – Track sales and communicate with buyers.
- 🚀 Reach thousands of buyers – Expand your business with ease.
Start selling today and grow your online business on Sharkbow! 🛒
Open Your Store 🚀 ✖🚀 What Can You Do on Sharkbow?
Sharkbow.com gives you endless possibilities! Explore these powerful features and start creating today:
- 📝 Create Posts – Share your thoughts with the world.
- 🎬 Create Reels – Short videos that capture big moments.
- 📺 Create Watch Videos – Upload long-form content for your audience.
- 📝 Write Blogs – Share stories, insights, and experiences.
- 🛍️ Sell Products – Launch and manage your online store.
- 📣 Create Pages – Build your brand, business, or project.
- 🎉 Create Events – Plan and promote your upcoming events.
- 👥 Create Groups – Connect and build communities.
- ⏳ Create Stories – Share 24-hour disappearing updates.
Join Sharkbow today and make the most out of these features! 🚀
Start Creating Now 🚀- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- الألعاب
- Gardening
- Health
- الرئيسية
- Literature
- Music
- Networking
- أخرى
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
