Cell and gene therapy CRO market, Size and Share by 2035
The “Cell and Gene Therapy CROs Market, 2022-2035” report features an extensive study of the current market landscape, offering an informed opinion on the likely adoption of these therapies over the next decade.
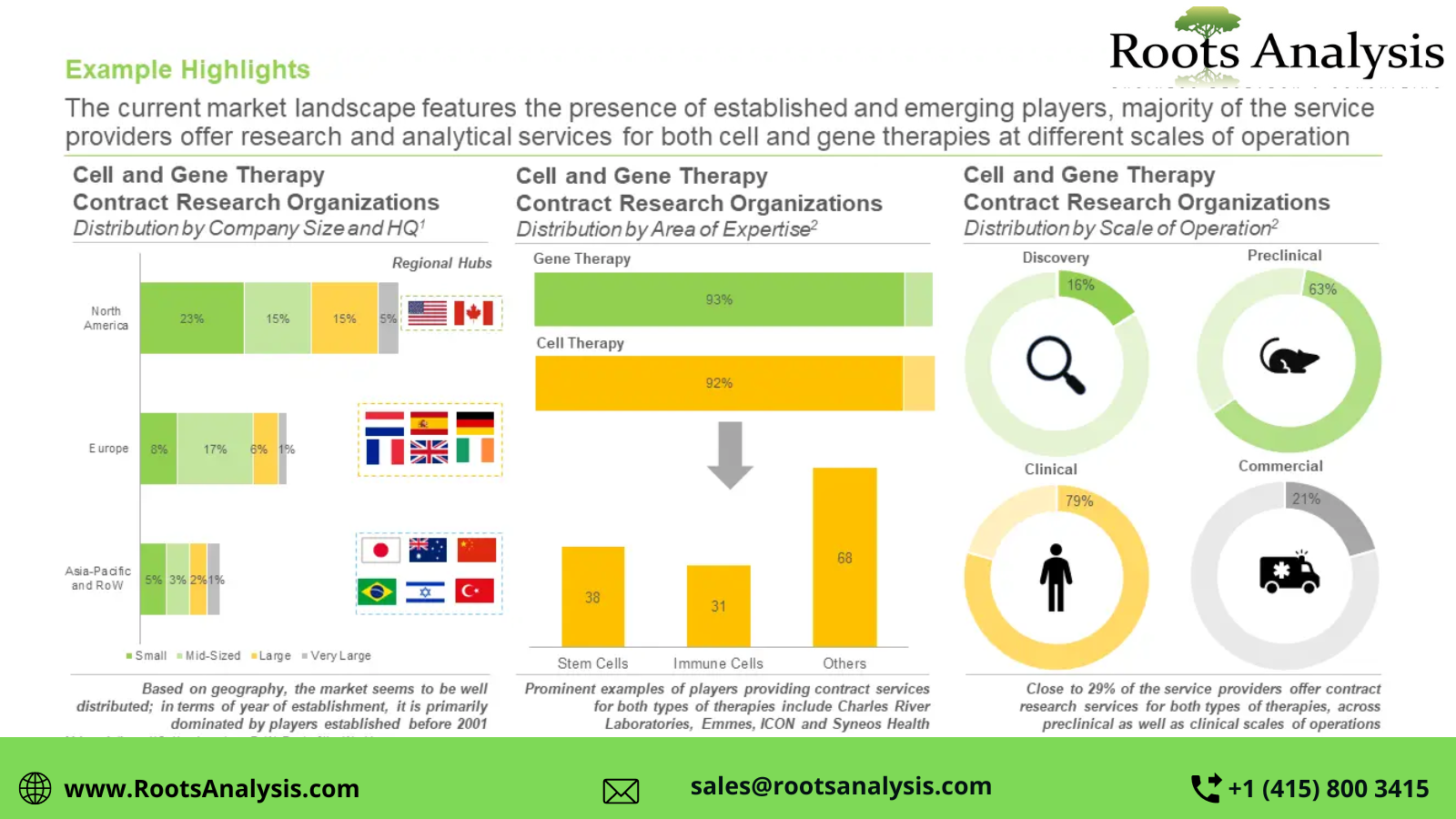
Roots Analysis is pleased to announce the publication of its recent study, titled, “Cell and Gene Therapy CROs Market, 2022-2035”.
Key Inclusions
§ A detailed overview of the overall market landscape of the cell and gene therapy CROs, based on several relevant parameters, such as year of establishment, company size (in terms of number of employees), location of headquarters, area of expertise (cell therapy (stem cells, T cells, dendritic cells, NK cells and tumor cells) and gene therapy), scale of operation (discovery, preclinical, clinical and commercial) and types of services offered, including [A] preclinical services (bioanalytical services, in-vivo studies, pharmacokinetic and ADME services, preclinical safety studies, toxicology studies and other preclinical services), [B] clinical services (clinical research monitoring, clinical trial management/ clinical project management, data management, safety and pharmacovigilance, and other clinical services), [C] regulatory services (GAP analysis, IND preparation, legal representation and technical dossier submission) and [D] general support services (biostatistics, consulting, post-market assessment, re-imbursement and training).
§ A detailed analysis on the business models that are commonly adopted by the biopharmaceutical industry for outsourcing cell and gene therapies. In addition, it includes information on various factors that drive developers towards outsourcing and key parameters that sponsors must consider while choosing CROs at each phase of drug development process.
§ Elaborate profiles of key industry players (very large and large companies) based in North America, Europe and Asia-Pacific that offer contract research services for both cell and gene therapies across both preclinical and clinical scales of operation. Each profile features a brief overview of the company, along with details related to its cell and gene therapies-related service portfolio, recent developments, and an informed future outlook.
§ A benchmark analysis of the various players engaged in this domain. It highlights the capabilities of the companies (in terms of their expertise across various services related to the development of cell and gene therapies). The analysis allows companies to compare their existing capabilities within and beyond their peer groups and identify opportunities to gain a competitive edge in the industry.
§ An analysis of the recent collaborations within the cell and gene therapy contract research industry, based on several relevant parameters, such as year of partnership, type of partnership, area of expertise, most active players (in terms of number of deals inked) and regional distribution of partnership activity that have been undertaken in this domain, during the period 2015-2022.
§ A detailed analysis of the various mergers and acquisitions that have taken place in this domain, during the period 2015-2022, based on several parameters, such as year, type of agreement, area of expertise, geographical location of the companies and key value drivers.
§ A detailed acquisition target analysis, taking into consideration historical trend of the activity of players that have acquired other firms since 2015, and offering a means for other industry stakeholders to identify potential acquisition targets.
§ A list of over 310 cell therapy developers that are anticipated to partner with cell therapy CROs. These players have been shortlisted based on several relevant parameters, such as developer strength (based on company's size and its experience in this field), pipeline strength and maturity (based on the number of pipeline drugs and affiliated stage of development), and availability of other cell therapy capabilities.
§ An in-depth analysis of nearly 235 gene therapy developers that are anticipated to partner with gene therapy CROs and have been shortlisted on the basis of several relevant parameters, such as developer strength (based on company's size and its experience in this field), pipeline strength and maturity (based on the number of pipeline drugs and affiliated stage of development) and availability of other gene therapy capabilities.
§ An in-depth analysis of completed, ongoing, and planned clinical studies of various cell and gene therapies, based on several relevant parameters, such as trial registration year, phase of development, current trial status, enrolled patient population, study design, leading industry players (in terms of number of trials conducted), therapeutic area and key geographical regions.
§ A detailed analysis of the total cost of ownership for large / very large cell and gene therapy contract research organizations. It features an informed estimate of direct and indirect expenses taking into consideration various relevant parameters, over a span of 20 years.
§ A detailed discussion on affiliated trends, key drivers and challenges, under a SWOT framework, which are likely to impact the industry's evolution highlighting the relative effect of each SWOT parameter on the overall cell and gene therapies research services industry.
The report also features the likely distribution of the current and forecasted opportunity across important market segments, mentioned below:
§ Area of Expertise
§ Cell Therapy
§ Gene Therapy
§ Scale of Operation
§ Preclinical
§ Clinical
§ Drug Discovery
§ Therapeutic Area
§ Oncological Disorders
§ Neurological Disorders
§ Cardiovascular Disorders
§ Infectious Diseases
§ Metabolic Disorders
§ Autoimmune Disorders
§ Blood Disorders
§ Rare / Genetic Disorders
§ Ophthalmic Disorders
§ Other disorders
§ Analysis by Geographical Regions
§ North America
§ Europe
§ Asia Pacific
§ Latin America
§ Middle East and North Africa
Key Questions Answered
§ Who are the leading players engaged in offering cell and gene therapy related research services?
§ Which business models are most commonly adopted by biopharmaceutical developers for outsourcing cell and gene therapies?
§ Which partnership models are commonly adopted by stakeholders engaged in this industry?
§ What are the key value drivers of the merger and acquisition activity in the cell and gene therapy market?
§ Which players are likely to partner with cell and gene therapy CROs?
§ Which regions emerged as the key hubs for carrying out clinical studies focused on cell and gene therapies?
§ How is the current and future market opportunity likely to be distributed across key market segments?
§ What are the anticipated future trends related to cell and gene therapy related research services?
To view more details on this report, click on the link:
https://www.rootsanalysis.com/reports/view_document/cell-and-gene-therapy-cros-market/230.html
News article
Non-viral Drug Delivery Systems Market
Learn from experts: do you know about these emerging industry trends?
mRNA Therapeutics and mRNA Vaccines Industry: Current Scenario and Future Trends
Learn from our recently published whitepaper: -
Next Generation Biomanufacturing – The Upcoming Era of Digital Transformation
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jocuri
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Alte
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
